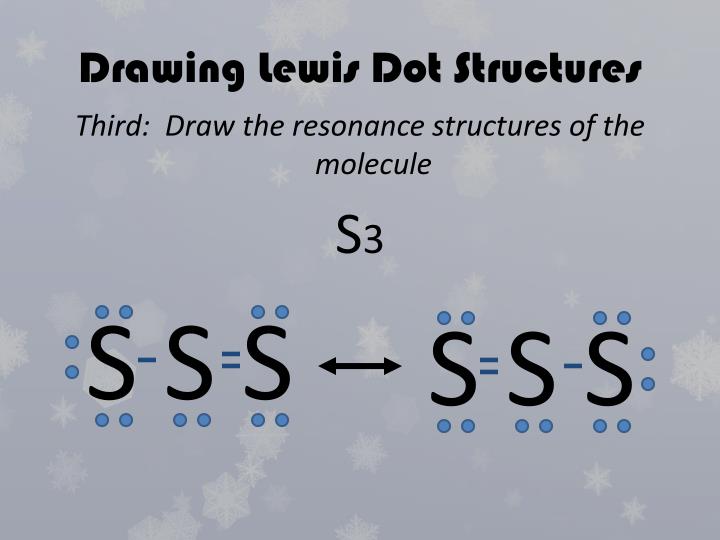
Does S3 Have Resonance . for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. what resonance structure is the most abundant in sulfur trioxide: Draw the lewis structure for the trisulfur (s3) molecule. Be sure to include all resonance structures that satisfy the octet rule. When you draw the lewis structure, you first get the three structures at the top. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. There are 2 steps to solve this one. The one with one double and two single, the one with two. In each of them, s has a. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. there are seven resonance structures for so3.
from www.slideserve.com
Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. When you draw the lewis structure, you first get the three structures at the top. Draw the lewis structure for the trisulfur (s3) molecule. The one with one double and two single, the one with two. There are 2 steps to solve this one. there are seven resonance structures for so3. what resonance structure is the most abundant in sulfur trioxide: In each of them, s has a. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or.
PPT Resonance Structures PowerPoint Presentation ID3080367
Does S3 Have Resonance In each of them, s has a. The one with one double and two single, the one with two. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. Draw the lewis structure for the trisulfur (s3) molecule. When you draw the lewis structure, you first get the three structures at the top. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. Be sure to include all resonance structures that satisfy the octet rule. there are seven resonance structures for so3. In each of them, s has a. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. what resonance structure is the most abundant in sulfur trioxide: There are 2 steps to solve this one.
From www.youtube.com
SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2 Does S3 Have Resonance there are seven resonance structures for so3. In each of them, s has a. There are 2 steps to solve this one. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. The one with one double and two single, the one with two. resonance occurs when we can draw two or more legitimate. Does S3 Have Resonance.
From irfanvanheek.blogspot.com
S3 Lewis Structure Resonance Lewis Structures Orbital Overlap Lewis Does S3 Have Resonance resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. When you draw the lewis structure, you first get the three structures at the top. Be sure to include all resonance structures that satisfy the octet rule. In each of them, s has a. there are seven resonance structures for so3. . Does S3 Have Resonance.
From www.researchgate.net
Fig. S3 Two types of resonance enhanced Raman scattering. (a) The pump Does S3 Have Resonance The one with one double and two single, the one with two. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. there are seven resonance structures for so3. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. Some. Does S3 Have Resonance.
From www.researchgate.net
Figure S3 Differential (on resonanceoff resonance) spectra as Does S3 Have Resonance The one with one double and two single, the one with two. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. Be sure to include all resonance structures that satisfy the octet rule. what resonance structure is the most abundant in sulfur trioxide: for example ch 3 cno can be. Does S3 Have Resonance.
From codehealth.io
S3 Heart Sound Does S3 Have Resonance for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. The one with one double and two single, the one with two. what resonance structure is the most abundant in sulfur trioxide: In each of them, s has a. Draw the lewis structure for the trisulfur (s3). Does S3 Have Resonance.
From www.showme.com
Lewis dot structuresresonance Science ShowMe Does S3 Have Resonance When you draw the lewis structure, you first get the three structures at the top. there are seven resonance structures for so3. The one with one double and two single, the one with two. what resonance structure is the most abundant in sulfur trioxide: Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures.. Does S3 Have Resonance.
From www.researchgate.net
Figure S3 Measured response of the resonance at 1548.3652 nm which is Does S3 Have Resonance there are seven resonance structures for so3. Draw the lewis structure for the trisulfur (s3) molecule. what resonance structure is the most abundant in sulfur trioxide: In each of them, s has a. Be sure to include all resonance structures that satisfy the octet rule. resonance occurs when we can draw two or more legitimate lewis structures. Does S3 Have Resonance.
From chemwiki.ucdavis.edu
Resonance Chemwiki Does S3 Have Resonance for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. what resonance structure is the most abundant in sulfur trioxide: Draw the lewis structure for the trisulfur (s3) molecule. Be sure to include all resonance structures that satisfy the octet rule. The one with one double and. Does S3 Have Resonance.
From www.pinclipart.com
Image Showing Resonance Strcuture Of So3 So3 Resonance Structures Does S3 Have Resonance Be sure to include all resonance structures that satisfy the octet rule. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. There are 2 steps to solve this one. what resonance structure is the most abundant in sulfur trioxide: The one with one double and two. Does S3 Have Resonance.
From byjus.com
How many resonance structures does N3 have? Does S3 Have Resonance Be sure to include all resonance structures that satisfy the octet rule. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. In each of them, s has a. When you draw the lewis structure, you first get the three structures at the top. resonance occurs when. Does S3 Have Resonance.
From aunitedkingdomfilm.com
S3 Lewis Structure Resonance Does S3 Have Resonance Be sure to include all resonance structures that satisfy the octet rule. Draw the lewis structure for the trisulfur (s3) molecule. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. The one with one double and two single, the one with two. there are seven resonance structures for so3. for. Does S3 Have Resonance.
From loveelectronics.blogspot.com
LoveElectronics RESONANCE WAVES, ELECTRICITY AND Does S3 Have Resonance In each of them, s has a. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. The one with one double and two single, the one with two. There are. Does S3 Have Resonance.
From ar.inspiredpencil.com
S3 Lewis Structure Does S3 Have Resonance what resonance structure is the most abundant in sulfur trioxide: When you draw the lewis structure, you first get the three structures at the top. There are 2 steps to solve this one. In each of them, s has a. The one with one double and two single, the one with two. Some molecules have two or more chemically. Does S3 Have Resonance.
From www.animalia-life.club
So3 Resonance Structures Does S3 Have Resonance In each of them, s has a. There are 2 steps to solve this one. The one with one double and two single, the one with two. Draw the lewis structure for the trisulfur (s3) molecule. When you draw the lewis structure, you first get the three structures at the top. there are seven resonance structures for so3. . Does S3 Have Resonance.
From www.researchgate.net
Figure S3. Resonance frequencies of LSPhs from a sampling of 55 Does S3 Have Resonance The one with one double and two single, the one with two. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. In each of them, s has a. there are seven resonance structures for so3. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. There are. Does S3 Have Resonance.
From irfanvanheek.blogspot.com
S3 Lewis Structure Resonance Lewis Structures Orbital Overlap Lewis Does S3 Have Resonance Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. there are seven resonance structures for so3. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. Be sure to include all resonance structures that satisfy the octet rule. The one with one. Does S3 Have Resonance.
From courses.lumenlearning.com
How to Draw Resonance Contributors MCC Organic Chemistry Does S3 Have Resonance When you draw the lewis structure, you first get the three structures at the top. The one with one double and two single, the one with two. In each of them, s has a. for example ch 3 cno can be represented by at least three different but valid lewis structures called resonance forms, or. Draw the lewis structure. Does S3 Have Resonance.
From ar.inspiredpencil.com
S3 Lewis Structure Does S3 Have Resonance In each of them, s has a. Draw the lewis structure for the trisulfur (s3) molecule. When you draw the lewis structure, you first get the three structures at the top. resonance occurs when we can draw two or more legitimate lewis structures for the same molecule. what resonance structure is the most abundant in sulfur trioxide: . Does S3 Have Resonance.